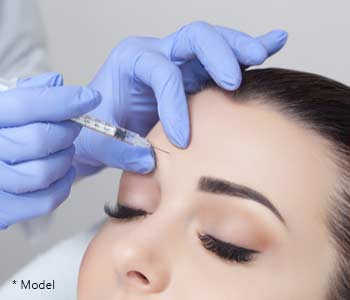
Botox is one of the most common minimally invasive (non-surgical) procedures used for facial rejuvenation. In fact, Botox was used nearly 2.5 million times, in 2010, by plastic surgeons. Botox (Botulinum toxin) is a neurotoxin that is injected into very specific areas of the face to weaken certain facial muscles. By weakening these muscles, we can reduce the appearance of wrinkles that are caused by long term facial muscle actions. The idea is not to totally paralyze the face and create a frozen look, but rather to soften facial movements and reduce wrinkles. With weaker muscles, it will be more difficult for you to make certain facial expressions. This will essentially retrain you to avoid making these expressions and prevent wrinkles.
Botox is extremely effective for reducing vertical furrows between the eyebrows, and transverse wrinkles at the bridge of the nose. It is also used to reduce the transverse wrinkles on your forehead and crow’s feet around your eyes. Botox can also create a mild brow lift to make you look less tired.
The effect of Botox is temporary. Most people can expect to notice the effects for about 3-4 months, at which point they will need repeated treatment. With regular treatments started early, we may be able to prevent the formation of certain wrinkles.
Dermal fillers can also be used as a non-surgical technique for facial rejuvenation. These act very different from Botox, as they are used to fill areas of soft tissue deficiency and also to fill wrinkles, and they do not weaken your facial muscles. Fillers are used in conjunction with Botox to fill areas that Botox will not be able to treat.
Examples of facial fillers include: Juvederm, Radiesse, and Sculptra.
The type of filler used, is determined by the location of treatment area, and the length of desired result.
BOTOX® Cosmetic (onabotulinumtoxinA) Important Information
Indication
BOTOX® Cosmetic is indicated for the temporary improvement in the appearance of moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity in patients 18 to 65 years of age.
IMPORTANT SAFETY INFORMATION, INCLUDING BOXED WARNING
CONTRAINDICATIONS
BOTOX® Cosmetic is contraindicated in the presence of infection at the proposed injection site(s) and in individuals with known hypersensitivity to any botulinum toxin preparation or to any of the components in the formulation.
WARNINGS
The recommended dosage and frequency of administration for BOTOX® Cosmetic should not be exceeded. Risks resulting from administration at higher dosages are not known.
The potency Units of BOTOX® Cosmetic are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products and, therefore, Units of biological activity of BOTOX® Cosmetic cannot be compared to or converted into Units of any other botulinum toxin products assessed with any other specific assay method.
Please refer to Boxed Warning for Distant Spread of Toxin Effect.
No definitive, serious adverse event reports of distant spread of toxin effect associated with dermatologic use of BOTOX® Cosmetic at the labeled dose of 20 Units (for glabellar lines) have been reported.
Serious and/or immediate hypersensitivity reactions have been reported. These reactions include anaphylaxis, urticaria, soft-tissue edema, and dyspnea. If such reactions occur, further injection of BOTOX® Cosmetic should be discontinued and appropriate medical therapy immediately instituted. One fatal case of anaphylaxis has been reported in which lidocaine was used as the diluent and, consequently, the causal agent cannot be reliably determined.
Individuals with peripheral motor neuropathic diseases, amyotrophic lateral sclerosis, or neuromuscular junctional disorders (eg, myasthenia gravis or Lambert-Eaton syndrome) should be monitored particularly closely when given botulinum toxin. Patients with neuromuscular disorders may be at increased risk of clinically significant effects including severe dysphagia and respiratory compromise from typical doses of BOTOX® Cosmetic.
This product contains albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases. A theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD) also is considered extremely remote. No cases of transmission of viral diseases or CJD have ever been identified for albumin.
PRECAUTIONS
Caution should be used when BOTOX® Cosmetic treatment is used in patients who have an inflammatory skin problem at the injection site, marked facial asymmetry, ptosis, excessive dermatochalasis, deep dermal scarring, thick sebaceous skin, or the inability to substantially lessen glabellar lines by physically spreading them apart.
Patients should be counseled that if loss of strength, muscle weakness, or impaired vision occur, they should avoid driving a car or engaging in other potentially hazardous activities.
Co-administration of BOTOX® Cosmetic and aminoglycosides or other agents interfering with neuromuscular transmission (eg, curare-like nondepolarizing blockers, lincosamides, polymyxins, quinidine, magnesium sulfate, anticholinesterases, succinylcholine chloride) should only be performed with caution as the effect of the toxin may be potentiated.
The effect of administering different botulinum neurotoxin serotypes at the same time or within several months of each other is unknown. Excessive neuromuscular weakness may be exacerbated by administration of another botulinum toxin prior to the resolution of the effects of a previously administered botulinum toxin.
Administration of BOTOX® Cosmetic is not recommended during pregnancy. There are no adequate and well-controlled studies of BOTOX® Cosmetic in pregnant women.
It is not known whether BOTOX® Cosmetic is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when BOTOX® Cosmetic is administered to a nursing woman.
ADVERSE REACTIONS
The most serious adverse events reported after treatment with botulinum toxin include spontaneous reports of death, sometimes associated with anaphylaxis, dysphagia, pneumonia, and/or other significant debility.
There have also been reports of adverse events involving the cardiovascular system, including arrhythmia and myocardial infarction, some with fatal outcomes. Some of these patients had risk factors including pre-existing cardiovascular disease.
The most frequently reported adverse events following injection of BOTOX® Cosmetic include blepharoptosis and nausea.
Excessive doses of BOTOX® Cosmetic may be expected to produce neuromuscular weakness with a variety of symptoms. Respiratory support may be required where excessive doses cause paralysis of respiratory muscles. In the event of overdose, the patient should be medically monitored for symptoms of excessive muscle weakness or muscle paralysis.
In the event of suspected or actual overdosage, please contact your local or state health department to process a request for antitoxin through the Centers for Disease Control and Prevention (CDC). If you do not receive a response within 30 minutes, please contact the CDC directly at 1-770-488-7100.
Please see BOTOX® Cosmetic full Prescribing Information including Boxed Warning and Medication Guide.
JUVÉDERM® XC Important Safety Information

JUVÉDERM® injectable gel (including JUVÉDERM® Ultra, JUVÉDERM® Ultra Plus, JUVÉDERM® Ultra XC, and JUVÉDERM® Ultra Plus XC) is indicated for correction of moderate to severe facial wrinkles and folds (such as nasolabial folds).
Treatment side effects are usually mild to moderate, lasting 7 days or less, and include temporary injection-site reactions such as: redness, pain, firmness, swelling, and bumps. JUVÉDERM® is not for people with severe allergies.
For more information, please click on the About Safety link at www.juvederm.com or call the Allergan Product Support line at 1-877-345-5372. JUVÉDERM® injectable gel is available by prescription only.